1. General Information
Welcome to the School of Medical Sciences
Welcome to your Postgraduate Taught Programme in the School of Medical Sciences within the Faculty of Biology, Medicine and Health at the University of Manchester. The University has a worldwide reputation based on high quality teaching and research, and I am sure that your programme will provide a solid foundation for your future career success.
Within the School and the wider Faculty, our goal is to create an environment that allows you to excel and reach your full potential. Offering access to first-class facilities and strong links with regional health-service providers, our postgraduate programmes are designed to meet the diverse needs of all our students. The curriculum of our taught programmes provides the knowledge and skills you will need in your subject area and all our Masters programmes include an opportunity to carry out an independent research project on topics spanning all areas of biomedical research from molecular to experimental biology and clinical medicine. While subject areas cover a broad range, all our taught programmes have two common aims:
- To develop your skills in your chosen field of study
- To enhance your knowledge within the field you have chosen. Whether you are a graduate, professional or have a clinical background, the programmes have been tailored to meet your specific needs.
As a student of the School of Medical Sciences, you will be expected to take responsibility for your degree, within a supportive environment that fosters your development and helps prepare you for your future career. This handbook will be a useful resource as you progress through your programme. It provides programme-specific information that I am sure that you will find helpful throughout your study. If however, you have questions or would like some further advice, please do not hesitate to contact the people listed in this handbook for further information and assistance.
I wish you every success as you embark upon your programme, and in your future career.
Dr Carol Yates
Director of Postgraduate Taught Education
School of Medical Sciences
Faculty of Biology, Medicine and Health
Welcome to the MRes Experimental Medicine
At a joint meeting entitled “Ensuring UK leadership in experimental medicine” given by the Association of the British Pharmaceutical Industry (ABPI) and the British Pharmaceutical Society (BPS) in October 2014 Sir John Savill, Chief Executive of the MRC reported: “Central to experimental medicine is the need for a skilled workforce able to design and lead early clinical studies, as well as the infrastructure and personnel required to deliver high-quality studies to industry-relevant timescales”
This MRes degree in Experimental Medicine is an integral part of the strategic plan development of Experimental Medicine in Greater Manchester and more broadly across the UK. It is currently the only UK course to provide the opportunity for students to gain experience through learning whilst being embedded in clinical research teams.
The objective of the course is to enable students to gain skills in designing and leading high quality early clinical studies and to ensuring delivery to industry-relevant timescales. Three principle methods will be used to achieve this:
• Taught units: Four compulsory taught units will provide the theoretical framework to understand experimental medicine
• Dissertation: Two six month projects will enable students to design, deliver and interpret projects related to experimental medicine
• Experience: As members of Experimental Medicine Teams, students will become part of the skilled workforce designing and contributing to early clinical studies
As programme director for the MRes in Experimental Medicine, I would like to formally welcome you onto the programme, and also take the opportunity to invite you to contact me should any concerns or clarifications arise during your graduate learning experience. The Pathway Lead is also available to offer you specific support during your studies (please see below for contact details).
List of important contacts
List of important contacts with e-mail, telephone and location
| Programme Directors: | Programme Director Professor Gillian Wallis The University of Manchester Division of Medical Education Room 1.008 , Core Technology Facility Manchester M13 9NT Tel: 0161 701 1263 g.wallis@manchester.ac.ukPathway Lead – Dermatology Prof Rachel Watson University of Manchester 1.721 Stopford Building Oxford Road Manchester M13 9PT Tel: 0161 275 5505 Rachel.Watson@manchester.ac.ukPathway Lead – Hearing Health Dr Kai Uus University of Manchester B2.1 in Ellen Wilkinson Building Oxford Road Manchester M13 9PT Tel: 0161 275 3373 kai.uus@manchester.ac.uk Pathway Lead – Musculoskeletal Pathway Lead – Respiratory |
| Programme Administrator:
MRes Experimental Medicine Student Representative |
Christie Finegan Programme Administrator University of Manchester Room 1.485 Stopford Building Oxford Road Manchester M13 9PT e-mail: cancer@manchester.ac.uk or Christie.finegan@manchester.ac.uk To be appointed by students post registration. The student rep will be nominated from the selection of the students who would like to volunteer for the position. The student rep is required to feedback to the programme directors and administrator on any issues or queries that the students have. They will be required to attend official programme committees throughout the year. |
| eLearning Support: | You can contact eLearning to receive support at elearning@manchester.ac.uk. However, you must enter “FBMH eLearning – MRes Experimental Medicine” in your email subject header. This will help ensure your request reaches us quickly. Further information on BMH eLearning can be found here. |
| General IT Support: | Contact the Service Desk via the online Knowledge Base http://servicedesk.manchester.ac.uk/portal/ss and report any IT failure or submit a service request. Call the Service Desk on: 0161 306 5544 (or ext 65544). Opening hours are: Monday to Friday 9am to 5pm. |
Online Skills Training Resource
The Faculty has developed a skills training resource to support you through your postgraduate taught programme. This online material should supplement the assessed learning material and activities undertaken in your taught programme.
Accessing the online skills resource
You can access Blackboard through the My Manchester portal (http://my.manchester.ac.uk). The skills training resource is available in an academic community space available to all registered PGT students in the Faculty through Blackboard.
If you cannot see these units in your Blackboard please contact your Programme Administrator.
Content
Full details of all these resources can be found in the introduction to each unit. These resources have been designed to give you formative feedback on your progress through them. If you experience any problems and would like to talk to someone please contact your Programme Director. If you have questions about referencing and how it applies to your own work, please contact your Programme Director or dissertation supervisor/module lead.
| Academic Writing | This is an excellent resource that supports you to write your assignments and dissertation. It is split into units that focus on key areas that previous students have found difficult and aims to enhance your academic writing style. |
| Research Methods* | This course is spilt into 3 units that cover introductions to study design, statistics and dissertation skills. It has a number of online quizzes where you can test your knowledge. |
| Statistics* | The course provides a valuable foundation for understanding and interpreting biostatistics. It aims to provide you with the fundamentals of quantitative analysis. |
| Presentation Skills | This short interactive unit is designed to help you to enhance your presentation skills. Regardless of whether you are presenting in public, preparing for conferences, an oral examination or more informal settings this unit will give you the tops tips to improve your delivery. |
| Qualitative Research Methods* | This unit has been designed to give you an introduction to Qualitative Research. |
| SPSS* | This is an introduction to statistics, using SPSS, a popular and comprehensive data analysis software package containing a multitude of features designed to facilitate the execution of a wide range of statistical analyses. |
| Intellectual Property Awareness Resource | This Intellectual Property (IP) awareness resource has been created in order to improve your understanding of IP. Topics include: Types of intellectual property • Copyright and IP clearance • University policy on IP • IP commercialisation • IP in research or consultancy • IP issues to be aware when dealing with academic materials |
* NOTE: the material in this online resource is for reference and formative learning purposes only. In some of your taught programme you may be required to undertake assessed course units for Research Methods, Qualitative Research or Statistics. If your programme involves taught units then you should refer to the Blackboard material relating to that course unit. Please contact your Programme Administrator if you are unsure which material relates to your assessed work. You will still be able to refer to the online skills resource in later years.
Introductory Courses
All students are automatically enrolled onto a number of introductory course units that provide information on health and safety, academic malpractice and academic literacy. Completion instructions for each of these units are clearly defined within the course. Completion of these units is mandatory for all students. All assessments must be completed as soon as possible after the programme begins, with the academic malpractice assessment completed before the first piece of coursework is submitted. Completion of these units is monitored by the School.
Health and Safety
Before you visit the University campus, please take time to read the University’s Health and Safety Policy. This can be accessed via: http://documents.manchester.ac.uk/display.aspx?DocID=654
2. Overview of the Programme
This programme offers both part-time and full time pathways. The structure of the programme is as follows:
PG Cert Experimental Medicine
Complete the 4 course units indicated below. These units together contribute 60 credits to the programme.
Course Units
Unit 1: Research Methods MEDN 69910 (15 credits)
Unit 2: Introduction to Experimental Medicine MEDN 69631 (15 credits)
Unit 3: Assembling pre-clinical and early clinical development strategies for a new candidate drug MEDN 66212 (15 credits)
Unit 4: Designing a translational medicine strategy for a clinical intervention MEDN69632 (15 credits)
MRes Experimental Medicine – 1 year full time or 3 years part time
Under supervision, students will produce 2 research dissertations (MEDN66230 and MEDN 66242). This will involve identifying research questions, developing the design of a research project, carrying out the research, analysing the data and then writing up the project. This will usually be in the format of a report based dissertation or systematic review. Submission in another format may be permitted by prior approval by the Programme Director.
Progression between stages is subject to satisfactory performance. You will not be able to progress to the dissertation unless you have successfully completed 120 credits at Masters level.
Aims and Learning Outcomes
Overall this programme aims to present an integrated and multi-disciplinary programme that will equip the student with the knowledge, understanding and core skills to practice and/or conduct research in an experimental medicine centre within the healthcare sector.
The programme aims to
01. Provide students with the key background knowledge and understanding of experimental medicine clinical trials
02. Train students to understand the pre-clinical data which is required before human testing of investigational therapeutic products can commence
03. Introduce key technical skills in interpretation of pharmacokinetic (including absorption, distribution, metabolism, elimination – ADME), pharmacodynamic and safety data which arise during early phase clinical trials
04. Emphasise the breadth and depth of the application methods used to design an early phase clinical programme of studies to determine whether to progress into later phase clinical testing
05. Comprehend key concepts and distinctions of the disciplines that need to be synthesised for conducting an effective early phase clinical trial
06. Provide an appreciation of the role of the experimental medicine scientist and how they fit in the wider landscape of therapeutic developments
07. Equip students with transferable clinical research methods
08. Embed the importance of patient-focused delivery and outcomes
Additionally for the Masters and PG Diploma programmes, it aims to
09. Equip students with in-depth knowledge, understanding and analytical skills to be able to work with data from experimental medicine trials to adapt the clinical trial, the clinical programme or to close the clinical trial and or clinical programme
10. Equip students with a systematic and critical understanding of relevant knowledge, theoretical frameworks and analytical skills to determine whether the pre-clinical package of information is sufficient to warrant early clinical trials
11. Develop a practical understanding of the skills, tools and techniques necessary to design first-in-human clinical trials
12. Apply practical understanding and skills to setting up, conducting and closing out a clinical study or clinical programme
13. Train students to be able to work in a multi-disciplinary community and communicate specialist knowledge of how to use data in a diverse community
14. Evaluate the effectiveness of alternative clinical trial and clinical programme designs with respect to time, cost and uncertainty of understanding
15. Enable students through the systematic, in-depth exploration of a specific area of experimental medicine practice and/or research to extend their knowledge, understanding and ability to contribute to the advancement of experimental medicine delivery, research or practice
Intended Learning Outcomes of the Programme
A. Knowledge & Understanding
Within the context of their chosen award students should:
For the Postgraduate Certificate
Identify pre-clinical data required by regulatory authorities in order to approve experimental medicine trials to commence
Demonstrate a critical understanding of the nature and value of different research approaches to the design of a first-in-human experimental medicine trial
Have knowledge and understanding of how to qualify and validate pharmacodynamics and predictive biomarkers
Appreciate and be able to discuss whether further investment to progress a drug or alternate therapy into expensive late phase clinical testing is warranted on the basis of data from experimental medicine trials
Awareness of national and international frameworks, strategies and policies to set up and run an experimental medicine trial
Understand the role of the experimental medicine scientist in early phase clinical development
Acquire knowledge of the history and current status of precision medicine
Understand the activities required to set up, recruit to and close a clinical study
Identify and explain the appropriate data sources to support approval for a clinical trial
Understand widely used pharmacokinetic principles to explore the anticipated drug exposure on an individual and population basis
Know how to formulate a translational strategy for a novel therapeutic intervention
Understand the range of statistical techniques to construct robust go/no go criteria
Additionally for the Masters and PG Diploma programmes
Have advanced knowledge of clinical trial designs aimed at testing a therapeutic intervention
Understand how an experimental medicine scientist fits in the organisational model for an experimental medicine centre
Systematically and critically examine hierarchies of research evidence that inform and underpin human experimental medicine clinical trials
Consolidate, synthesise and critically apply the in-depth knowledge and understanding of relevant policy, literature, methodologies and technologies acquired through the taught components of the programme to the formulation of an extended research project relevant to a specific aspect of experimental medicine
B. Intellectual Skills
At the end of the programme students should be able to:
For the Postgraduate Certificate
Appraise and synthesise information from a variety of sources in order to develop a coherent critical analysis of issues relating to testing therapeutic interventions in human subjects
Recognise problems and devise appropriate solutions
Translate knowledge and understanding obtained from other disciplines and make connections to experimental medicine
Critically analyse and summarise others and your own work and consider how it could have been done differently
Engage in a literature research strategy and be able to summarise key points
Additionally for the Masters and PG Diploma programmes
Consider critically a variety of established techniques and methods of research and enquiry and how they relate to the advancement of the testing of novel therapeutics
Critically evaluate a range of possible options or solutions to construct a clinical trial design and present a soundly reasoned justification of a final recommendation
Translate ideas, practices, knowledge and understanding between academic disciplines in order to provide innovative solutions to unfamiliar problems
Demonstrate original independent thinking, and an ability to develop theoretical concepts
Justify the methodologies, techniques and decisions used in one’s own work
Engage in a systematic exploration of the literature, policy and research related to a specific area of experimental medicine so as to demonstrate an in-depth understanding of the relationship between theory and practice
Understand relevant research methodologies and techniques and their appropriate application to research
C. Practical Skills
At the end of the programme students should be able to:
For the Postgraduate Certificate
Perform independent information acquisition and management
Critically appraise all aspects of experimental medicine protocol development
Additionally for the Masters
Draw on their knowledge and understanding of different approaches to research to formulate appropriate questions and methods for research and/or evaluation into aspects experimental medicine
Utilise diverse data sources and select key data to enable the clinical trial to be designed in a manner which mitigates risks and seeks to maximise understanding of the potential of a new therapeutic intervention
Plan, execute and evaluate experimental medicine clinical studies
Organise and pursue a scientific research project in a specific aspect surrounding experimental medicine
D. Transferable Skills and Personal Qualities
At the end of the programme students should be able to:
For the Postgraduate Certificate
Prepare, present and effectively communicate and defend complex ideas in documents and oral presentations to a range of audiences
Apply statistical skills
Demonstrate research and enquiry skills by accessing and analysing literature in order to inform, research and develop strategies
Work cooperatively and effectively with others as a member of a team
Reflect on their own academic performance and utilise strategies to improve these
Use logical and systematic approaches to problem-solving and decision-making
Work as an individual on self-directed learning and research
Transfer knowledge, understanding and skills to a range of experimental medicine settings
Manage resources and time effectively
Identify and access appropriate bibliographical resources, archives and other sources of relevant information to investigate a topic
Ability to make cross-disciplinary connections with other scientists and professionals
Additionally for the Masters and PG Diploma programmes
Show flexibility, open-mindedness, self-awareness, self-discipline, motivation and thoroughness to sustain a piece of research
Progression through the Programme
The diagram below shows progression through the programme, related to the semester dates. Students will be required to attend the allocated teaching days and to undertake the associated assessments. Progression from the PG Cert and the PG Dip will be dependent on successful completion of 60 credits, at the required standard. Similarly, progression from PG Dip to the MSc will depend on successful completion of 120 credits, at Masters Level.
| Units of Learning | Credits | Exit Award |
| 1. Taught Unit 1: Research Methods | 15 | |
| 2. Taught Unit 2: Introduction to Experimental Medicine | 15 | |
| 3. Taught Unit 3b: (Respiratory, Dermatology, Hearing Health and Musculoskeletal Pathways): Designing a translational medicine strategy for a clinical therapy or intervention | 15 | |
| 4. Taught Unit 4: Assembling pre-clinical and clinical strategies for a new candidate drug | 15 | PG Cert in Experimental Medicine (60 credit exit award for all pathways) |
| 1.Research Project 1 | 60 | PG Diploma in Experimental Medicine (120 credit exit award for all pathways – comprising Taught Units [60 credits] and Research Project 1 [60 credits]). |
| 2.Research Project 2 | 60 | MRes in Experimental Medicine (180 credits – comprising Taught Units [60 credits] and Research Projects 1 & 2 [120 credits]) |
Unit 1: Research Methods MEDN 69910 (15 Credits)
The Research Methods Course is a 15 credit, interactive blended learning course which provides students with an introduction to key material required for the design, execution and interpretation of medical, scientific and clinically-related research and the production of a high quality dissertation.
The unit will run online over one semester. There will be online material opened on each topic at specific timetabled slots throughout the semester and face-to-face consolidation sessions.
The unit incorporates online material and 10 face-to-face sessions covering:
Introduction to Blackboard and research methods online;
Dissertations skills – covering literature search, principles of academic writing, critical appraisal of publications, plagiarism and abstract writing;
Study design – covering project planning, time management and an overview of specific research designs, ethical issues and principles of good governance that apply to all clinical research;
Statistics – covering a basic introduction to statistical methods;
Communication skills – presentation skills and posters.
Unit 2: Introduction to Experimental Medicine MEDN 69631 (15 credits)
The Introduction to Experimental Medicine module is a 15 credit, interactive blended learning course which provides students with an overview of issues related to conducting EM research. This area of research is broad and the aim of this module is to give students a generic introduction to the concepts, practical issues, and challenges that researchers may experience when conducting EM research.
This module will cover basic concepts, ethical and regulatory considerations, study design, statistical methods, protocol development, and challenges encountered frequently in EM, including recruitment. The teaching will be delivered by clinical researchers active in EM research and will be supported by the Manchester Clinical Research Facilities.
Unit 3: Assembling pre-clinical and early clinical development strategies for a new candidate drug MEDN 66212 (15 credits)
The purpose of this unit is to provide a framework to consider how to optimise the chances of success in the early clinical testing of a novel intervention. Despite having a potentially excellent drug, testing it in the wrong disease type, or at the wrong schedule or dose; or with the wrong combination of agent- could terminate an otherwise promising drug. Students will gain a foundation for considering which pre-clinical experiments can be done to inform on the optimal options for clinical testing. With the help of a tutor, students will choose a scenario to the practice of relevant translational medicine within the drug development environment and constructively critique it.
Unit 4: Designing a translational medicine strategy for a clinical intervention MEN69632 (15 credits)
The purpose of this module is to provide a foundation and appreciation of what has to be considered in planning and designing the pre-clinical and early clinical strategy for a new candidate drug. A wide range of issues has to be considered before a drug is dosed in man. Amongst these is whether the pre-clinical toxicity supports entry into man, and if so, whether the first in man study should be performed in patients or healthy volunteers and what the initial starting dose should be, escalation dose increments and setting an upper dose limit?. A basic understanding of drug handling will also be provided, along with the pre-clinical pharmacokinetic and pharmaceutical development packages required to support an application to the regulatory authorities to gain approval to dose the drug in man. Key objectives of any early clinical development programme are the determination of (a) drug exposure- pharmacokinetics (b) drug safety (c) drug effect- pharmacodynamics and clinical efficacy. Understanding these three elements is critical if clear go/no-go criteria for progression of the drug into further clinical development are to be put in place and adhered to.
Unit 5: Research Project 1 MEDN 66230 (60 credits)
Under supervision, students will produce a research dissertation. This will involve identifying research questions, developing the design of a research project, carrying out the research, analysing the data and then writing up the project. This will usually be in the format of a report based dissertation or systematic review. Submission in another format may be permitted by prior approval by the programme director.
Unit 6: Research Project 2 MEDN 66242 (60 credits)
Under supervision, students will produce a research dissertation. This will involve identifying research questions, developing the design of a research project, carrying out the research, analysing the data and then writing up the project. This will usually be in the format of a report based dissertation or systematic review. Submission in another format may be permitted by prior approval by the programme director.
Course Unit Outlines
Unit 1: Research Methods MEDN 69910 (15 Credits)
The Research Methods Course is a 15 credit, interactive blended learning course which provides students with an introduction to key material required for the design, execution and interpretation of medical, scientific and clinically-related research and the production of a high quality dissertation.
The unit will run online over one semester. There will be online material opened on each topic at specific timetabled slots throughout the semester and face-to-face consolidation sessions.
The unit incorporates online material and 10 face-to-face sessions covering:
Introduction to Blackboard and research methods online;
Dissertations skills – covering literature search, principles of academic writing, critical appraisal of publications, plagiarism and abstract writing;
Study design – covering project planning, time management and an overview of specific research designs, ethical issues and principles of good governance that apply to all clinical research;
Statistics – covering a basic introduction to statistical methods;
Communication skills – presentation skills and posters.
2. AIMS
The unit aims to:
– introduce students to the skills and knowledge to critically design, effectively implement, ethically conduct and knowledgeably interpret research in Biomedical and Human Sciences.
– provide students with life-long critical appraisal skills that they will be able to apply to any research evidence that comes before them.
3. LEARNING OUTCOMES
On completion of this unit successful students will be able to:
| Knowledge and understanding |
|
| Intellectual skills |
|
| Practical skills |
|
| Transferable skills and personal qualities |
|
4. TEACHING AND LEARNING METHODS
The course unit will be delivered predominantly through e-learning over one semester, with seven timetabled face-to-face sessions run by the Graduate Training Team and three face-to-face sessions run by the Library Training Team. A variety of online material will be utilised including web-based reading, podcasts, online discussions, tutor feedback, interactive exercises, self-assessment through MCQs and self-reflection.
The timetabled sessions will be a mix of tutor presentations, group discussions and workshops.
5. ASSESSMENT METHODS
To help students appropriately focus their efforts throughout the unit, a combination of formative and summative assessments is offered.
All students must complete all formative assessments by the end of the semester.
Summative assessments will contribute a percentage towards the final mark. All students must complete three summative assessments in total. Two summative assessments “Critical appraisal of literature” and “Ethical issues related to clinical research” are compulsory for all programmes. Whereas each programme of study can choose the third summative assessment from three optional assessments: either Abstract writing or Grammar quiz or Statistics quiz.
6. FEEDBACK METHODS
Students will be provided with personalised feedback for their summative assignments, within 15 working days.
Unit 2: Introduction to Experimental Medicine MEDN 69631 (15 credits)
The Introduction to Experimental Medicine module is a 15 credit, interactive blended learning course which provides students with an overview of issues related to conducting EM research. This area of research is broad and the aim of this module is to give students a generic introduction to the concepts, practical issues, and challenges that researchers may experience when conducting EM research.
This module will cover basic concepts, ethical and regulatory considerations, study design, statistical methods, protocol development, and challenges encountered frequently in EM, including recruitment. The teaching will be delivered by clinical researchers active in EM research and will be supported by the Manchester Clinical Research Facilities.
2. AIMS
The unit aims to:
1. introduce students to general concepts of experimental medicine (EM) research
– understand the infrastructure and resource available to support EM research
– provide students with practical and real world understanding of the regulatory oversight of EM
– develop students’ understanding of ethical issues related to EM research, and introduce ethical considerations involved in special scenarios (e.g. children)
2. give students understanding of how to conduct EM research:
– develop an EM protocol and define research outcomes
– understand methodological issues and how they may be overcome
– awareness of clinical research facilities and how they support EM
– how to define populations of interest and choose appropriate inclusion/exclusion criteria
– understand how to plan data collection and statistical analysis in EM studies
3. provide students with an awareness of challenges in EM research:
– recruitment, retention, and healthy control populations
– how to develop collaborations
– how to translate EM into later phase research
– how to integrate public and patient involvement when planning EM studies
3. LEARNING OUTCOMES
| Category of outcome | Students should/will (please delete as appropriate) be able to: |
| Knowledge and understanding |
|
| Intellectual skills |
|
| Practical skills |
|
| Transferable skills and personal qualities |
|
4.TEACHING AND LEARNING METHODS
The course unit will be delivered through a mix of lectures, workshops, interactive group sessions and will be supported by e-learning opportunities. Presentations will be delivered by clinical researchers and staff involved in delivery of EM in Manchester, and will utilise the expertise of the NIHR Manchester Clinical Research Facilities (based at CMFT, UHSM and The Christie) to ensure students experience real-world EM examples to demonstrate key concepts. A variety of additional material and learning opportunities will be utilised to support the students’ learning, including journal articles and interactive exercises.
5. ASSESSMENT METHODS
| Assessment task | Length | Weighting within unit (if relevant) |
| Formative: tutor review and feedback from workshops on performance, engagement and content | Continuous through module | All formative |
| Assignment one: design patient information and consent forms to submit to ethics committee | 1000 words | Summative – 40% |
| Assignment two: design a methodology for running an EM study, to include experimental methods, outcomes, recruitment strategy and statistical design | 1500 words | Summative- 60% |
6. FEEDBACK METHODS
Students will be provided with personalised feedback for their summative assignments, within 15 working days.
Unit 3b: Respiratory, Dermatology, Hearing Health and Musculoskeletal Pathways – Designing a translational medicine strategy for a clinical intervention MEN69632 (15 credits)
The purpose of this unit is to provide a framework to consider how to optimise the chances of success in the early clinical testing of a novel intervention. Despite having a potentially excellent drug, testing it in the wrong disease type, or at the wrong schedule or dose; or with the wrong combination of agent- could terminate an otherwise promising drug. Students will gain a foundation for considering which pre-clinical experiments can be done to inform on the optimal options for clinical testing. With the help of a tutor, students will choose a scenario to the practice of relevant translational medicine within the drug development environment and constructively critique it.
2.AIMS
Equip students with knowledge and skills to
● Understand the principles of designing translational medicine strategies for a clinical intervention including application of statistical theory
● Gain comprehensive knowledge of the translational research cycle
● Understand how pre-clinical experiments should be conducted to inform the design of a first in human clinical trial
● Prioritise the most pertinent pieces of pre-clinical data to inform the design of a first in human clinical trial
● Explore baseline and outcome measures that define success of an intervention
● Prioritise in a resource constrained environment which pre-clinical experiments are critical to attaining the next milestone and enlightening the clinical plan beyond that milestone
● Understand how to interpret data from Phase 1 clinical studies including measures of safety and efficacy
3. LEARNING OUTCOMES
| Category of outcome | Students should/will (please delete as appropriate) be able to: |
| Knowledge and understanding | Understand the key design challenges for a first in human experiment
Explain the types of biomarkers and their application to drug development Understand the steps required to qualify and validate biomarkers Appreciate available pharmacodynamics biomarkers to demonstrate biological activity against the hallmarks of disease Understand how to interpret pre-clinical toxicological data to inform the safety design of the first-in-human study Level of evidence required to credential a putative target as meritorious of clinical testing Understand the research landscape for conducting experimental medicine studies |
| Intellectual skills | Explain how pre-clinical experiments can inform the key design challenges and thus optimise for success; yet balance the danger of over-interpreting pre-clinical data; identify the key data which governs whether a new drug should/should not progress into further clinical testing
Develop and communicate a strategy to recommend whether a dose escalation should/should not proceed in a first-in-human clinical trial Critically review which pre-clinical translational experiments most inform the clinical plan in a resource constrained environment Apply the framework of biomarker qualification to prioritise putative pharmacodynamics biomarkers from literature review |
| Practical skills | Perform and communicate how to assemble a translational strategy to support entry of a drug into man
Construct a schedule of assessments from a protocol synopsis |
| Transferable skills and personal qualities | Work collaboratively within a team
Present ideas and work in a verbal and written format Understand about resource allocation and project planning Identify and solve problems Reflect on your one’s response to both normal and complex situations demonstrating the professional behaviours and insights working within the limits of professional competence referring as appropriate to senior staff |
4. TEACHING AND LEARNING METHODS
This unit will use Project-Based Learning and will be delivered in a blended format combining independent directed and undirected reading, one-to-one tutorials and discussions to explore examples and encourage candidates to draw upon their own clinical experience and reading.
5. ASSESSMENT METHODS
| Assessment task | Length | How and when feedback is provided | Weighting within unit (if relevant) |
| Academic critical reflection on clinical testing of a novel drug | 2,000 words | Online | 100% |
6. FEEDBACK METHODS
Students will be provided with personalised feedback for their summative assignments, within 15 working days.
Unit 4: Assembling pre-clinical and early clinical development strategies for a new candidate drug MEDN 66212 (15 credits)
The purpose of this module is to provide a foundation and appreciation of what has to be considered in planning and designing the pre-clinical and early clinical strategy for a new candidate drug. A wide range of issues has to be considered before a drug is dosed in man. Amongst these is whether the pre-clinical toxicity supports entry into man, and if so, whether the first in man study should be performed in patients or healthy volunteers and what the initial starting dose should be, escalation dose increments and setting an upper dose limit?. A basic understanding of drug handling will also be provided, along with the pre-clinical pharmacokinetic and pharmaceutical development packages required to support an application to the regulatory authorities to gain approval to dose the drug in man. Key objectives of any early clinical development programme are the determination of (a) drug exposure- pharmacokinetics (b) drug safety (c) drug effect- pharmacodynamics and clinical efficacy. Understanding these three elements is critical if clear go/no-go criteria for progression of the drug into further clinical development are to be put in place and adhered to.
This unit will cover the following indicative content:
- An introduction to drug manufacture, formulation development, stability testing of the pharmaceutical product
- Pre-clinical pharmacology and toxicological studies pivotal to the design of the first in human experiment
- Basic pharmacokinetic parameters
- Understand the regulations surrounding first-in-human studies from the Expert Scientific Committee report from the TeGenero incident
- Inform students on how to conduct a site audit to appraise the feasibility of a clinical trials unit to conduct the study according to protocol
- Interpretation of tolerability data from an ascending dose Phase 1 trial in cancer patients, simulating a real-life dose escalation meeting
- Plot and interpret a pharmacokinetic drug concentration-time data from a Phase 1 trial
- Using real datasets to determine whether specific drugs should/should not progress into further clinical testing
2. AIMS
This unit aims to:
● Understand the safety, pharmacology and drug disposition data in animals which is required prior to commencing human clinical trials
● Understand the pharmaceutical manufacturing issues which need to be addressed to ensure the quality of the pharmaceutical product for human administration
● Understand the requirement for and basic design of pre-clinical safety studies required to assist clinicians in determining the likely range of safe exposures to the candidate drug, and the possible consequences if these doses are exceeded
● Provide practical guidance to balance the importance of a range of pre-clinical toxicities to safely entering man. If considered suitable for clinical testing, students will calculate the safe starting dose, dose increments and determine criteria for ceasing dose escalation
● Experience the platform of evidence data required to justify progressing a compound forward for further development incurring the significant resource and human exposure implications of the decision or whether to cease drug development
● Provide basic design considerations for first in human administration designs along with how to determine early tolerability, pharmacokinetics and efficacy of a candidate drug in the clinic
● Provide opportunity for students to learn the critical skill of site selection- to enhance the chance of a successful first-in-man study; assembling a site audit programme and then utilise this in a site visit to two first-in-human units to consider their suitability as a site
● Provide understanding of basic pharmacokinetic parameters
3. LEARNING OUTCOMES
| Category of outcome | Students should/will (please delete as appropriate) be able to: |
| Knowledge and understanding | Understand the pre-clinical package of information to support a MHRA application
Explain basic pharmacokinetic parameters and how these relate to safety and efficacy implications. Discuss issues surrounding whether or not to progress a drug into further development Appreciate data which is derived from each phase of clinical development Discuss the challenges in setting a safe starting dose Understand the role of quality manufacturing systems underpinning the pharmaceutical product Discuss and understand concepts for alternative designs of first-in-human trials |
| Intellectual skills | Identify the key data which governs whether a new drug should/should not progress into further clinical testing
Balance findings revealed during an audit of a clinical trials unit to inform the decision whether or not to place a clinical study Critically review a the recommendations from the Expert Scientifc committee report into the TeGenero incident and discuss the relevance to cancer trials Apply pharmacokinetic knowledge to dose setting decisions Design approaches and queries to retrieve data |
| Practical skills | Perform and communicate how to assemble a pre-clinical strategy to support entry of a drug into man
Construct a concentration-time plot and derive basic pharmacokinetic parameters |
| Transferable skills and personal qualities | Work collaboratively within a team
Present ideas and work in a verbal and written format Understand about resource allocation and project planning Work through the problem-solving cycle |
4. TEACHING AND LEARNING METHODS
This unit will delivered in a blended format combining face-to-face lectures and open discussions to introduce concrete examples and encourage attendees to draw upon their own reading and experience. Group, problem based learning will show a deeper understanding of the area and encourage collaborative working. Example case-studies will be drawn from real, anonymised datasets from first in human studies and drug development programmes. The F2F teaching will be delivered as ~20x 0.5-1.5h lectures over 2 weeks, and ~7 x 1.5–5h workshops. The workshops will allow the students (as groups) to actively participate in the different stages of pre-clinical and clinical development. The first will ask each student to individually prepare a proposal for a pre-clinical development package of a compound based on early physiochemical data and initial concepts for clinical testing. Students will be provided with a number of pre-clinical tests which can be conducted to support entry into man (and a limited budget which will not support conducting all tests) and will be required to prioritise those tests most informative to support the proposed clinical plan. In preparation for the second workshop, students will be provided with the Duff report from the TeGenero incident and each student will be allocated to defend the actions of one of the stakeholder groups. During the third workshop, data from a dose escalation cohort will be presented and discussed, simulating real-time dose escalation meetings. The limitations of differing definitions for “Dose limiting toxicity” will be demonstrated. The fourth workshop with introduce students to methods of plotting and interpreting a concentration-time profile on a semi-log plot, with calculation of basic PK parameters which can be used to calculate a loading dose and dosing interval. For the fifth workshop, students will be presented with data from the early clinical studies for several drugs and be asked to justify their rationale as to whether to continue or discontinue the drug from further development. A sixth workshop will consider what comprises a good phase I unit and features to audit- followed by a visit to 2 of the Manchester CRF’s to determine their suitability for conducting first in human studies. The seventh workshop will introduce students to the principles of human pharmacokinetics.
5. ASSESSMENT METHODS
| Assessment task | Length | How and when feedback is provided | Weighting within unit (if relevant) |
| WORKSHOP ASSIGNMENT 1: A written individual report to outline the strengths and liabilities of a site feasibility assessment with proposed mitigations
ASSIGNMENT 2: A written individual assessment and derivation of Pharmacokinetic parameters which can be measured in an experimental cancer medicine trial ASIGNMENT 3: A written individual report of a business case to support a pre-clinical and/or clinical capability build |
Maximum 1500 words
Maximum 1500 words Maximum 1500 words |
WRITTEN COMMENTS FROM 2 ASSESSORS VIA TURNITIN WITHIN 28DAYS OF SUBMISSION
WRITTEN COMMENTS FROM 2 ASSESSORS VIA TURNITIN WITHIN 28DAYS OF SUBMISSION WRITTEN COMMENTS FROM 2 ASSESSORS VIA TURNITIN WITHIN 28DAYS OF SUBMISSION |
33%
33% 33% |
| Formative.
Regular presentation of results to tutor and staff to elicit feedback and develop ideas/work. |
na | CONTINUOUS DURING MODULE BOTH ORAL AND WRITTEN | NA |
6. FEEDBACK METHODS
Students will be provided with personalised feedback for their summative assignments, within 15 working days.
3. Teaching, Learning and Assessment
Teaching and Learning Overview
The programme provides the necessary knowledge and understanding to work in experimental medicine. The programme aims to be problem-orientated. Students undertaking the full MRes programme will engage in experience-based learning so that on completing the programme they can take their newly acquired skills and knowledge and use them in other experimental medicine centres.
The course is designed to be flexible, to enable students to balance taught work with their research projects and by the integration into the Experimental Medicine Research Teams of the Manchester Biomedical Research Centre (BRC) (with 1 each for Hearing Health, Respiratory, Musculoskeletal and Dermatology). It is anticipated, given the interdisciplinary nature of the field, that students will come from a range of backgrounds, including physician, nursing, allied health professions and clinical trials administration. The programme will train them to be able to operate in an interdisciplinary environment using their specialist backgrounds, but also develop their understanding of the key concepts, methods and processes from other areas. It is this ability to work at the intersections of many disciplines that will highlight the unique position of the experimental medicine scientist.
Three taught units in the programme are core. The teaching on these units will be standard or a blend of F2F teaching and e-learning. The Faculty of Biology, Medicine and Health has extensive experience of blended learning which will be used in the first unit (Research Method course unit) and good practice in on-line learning with dedicated e-learning technologists and learning materials that include rich on-line audio/video/teleconference technologies; on-line problem/enquiry-based learning; interactive materials, exercises and self-assessment tools.
In order to complement on-line components of the course additional opportunities for F2F learning and networking between students, academic and relevant research staff within experimental medicine teams will be provided (particularly as elements of the course will be delivered on different sites such as the Clinical Research Facility, The Christie Hospital and at research centres based at the University and host Trusts of the BRC). This will allow students to be aware of the different settings for experimental medicine research.
A range of teaching and learning methods are used to facilitate achievement of unit and programme learning outcomes. More details on the specific methods are detailed in the individual unit specifications.
Postgraduate Taught Degree Regulations for Students
Postgraduate Taught degrees at the University of Manchester are based on the National Framework for Higher Education Qualifications (FHEQ). This framework requires students to achieve credit at masters’ level in order to get an award. For a standard postgraduate taught Masters programme this will normally mean passing 180 credits. A standard postgraduate diploma will normally have 120 credits and a postgraduate certificate 60 credits. The way in which you study these credits will be defined later in the programme handbook and the programme specification.
The University sets standards relating to your performance on every unit but also on your progression through the programme. The programme and course unit specifications will set out the requirements for passing the credit on individual units.
Postgraduate Taught Degree Regulations
Please find below the link to the degree regulations:
Postgraduate Taught Degree Regulations (for new PGT students registering from September 2016)
The following guidance should be read in conjunction with the Introduction to the Postgraduate Degree Regulations for Students:
http://www.tlso.manchester.ac.uk/degree-regulations/
Criteria for Awards
Award of Masters Degree
The award of Master degree is based upon credit accumulation using a pass mark of 50%.
Distinction
Exceptional achievements over the course of the Programme according to the taught masters marking scheme will be rewarded with the degree of Masters with Distinction.
To obtain a Distinction, students must have:
- accrued 180 credits;
- have passed all units with no compensations or referrals;
- have achieved an overall weighted average of 70% or more across the programme;
Students who have compensated or have been referred in any course units are not eligible for the award of Distinction. In addition, the dissertation must be submitted by the end of the period of programme, unless there are significant mitigating circumstances, approved in advance for missing the end of programme deadline.
Merit
To obtain a Merit, students must have accrued 180 credits AND have achieved an overall weighted average of 60% or more across the programme, including any provision made for compensated or referred units.
Pass
To obtain a pass, students must have accrued 180 credits including any provision made for compensated or referred units.
Progression
To progress to the dissertation / research element of the Masters programme, students must have passed all taught units (120 credits).
Award Postgraduate Diploma
To obtain a Postgraduate Diploma award, students must have accrued 120 credits (as specified by the programme) including any provision made for compensated or referred units.
Award Postgraduate Certificate
To obtain a Postgraduate Certificate award students must have accrued 60 credits (as specified by the programme) including any provision made for compensated or referred units.
Unless otherwise specified in the exemptions, the awards of Postgraduate Diploma and Postgraduate Certificate degree are based upon credit accumulation using a pass mark of 40% for which there is no classification other than pass/fail.
Exit Awards
Exit awards are available for students who do not satisfy the criteria for the programme they are registered on or who needs to exit the programme early due to unforeseen circumstances.
To be considered for a PG Diploma (120 credits; exit point) students must have accrued 120 credits across the programme.
To be considered for a PG Certificate (60 credits; exit point) students must have accrued 60 credits across the programme.
Please note the pass mark for course units making up the Postgraduate Diploma and Certificate exit awards is 40%.
Compensation
Masters Degree
Students may be awarded compensated credit if they receive fail marks in the range 40‑49% in no more than 30 credits in the taught component.
Postgraduate Diploma
Students may be awarded compensated credit if they receive fail marks in the range 30‑39% in no more than 30 credits in the taught component.
Postgraduate Certificate
Students may be awarded compensated credit if they receive fail marks in the range 30‑39% in no more than 15 credits in the taught component.
The combined total number of credits compensated and referred cannot exceed half the taught credits.
Compensated credit retains the original failed mark for the course unit and this is used in the weighted average for the calculation of the final classification/award.
Please note that some programmes do not allow compensation. Please refer to the ‘Programme Exemptions to PGT Degree Regulations’ section of the handbook where specific exemptions applicable to the programme will be listed.
Reassessment
Where the overall unit mark is below the compensation zone (40% for Masters and 30% for Postgraduate Diploma/Certificate) OR the number of compensatable fails (30 credits for Masters/Diploma and 15 credits for Postgraduate Certificate) has been exceeded, reassessment may be taken.
Reassessment as a result of a fail is known as a “Referral”. Reassessment as a result of approved and verified mitigating circumstances is known as “Deferral” and may be permitted where students are reassessed as a first attempt, for which no penalty applies.
Students may be referred in up to half of the total taught credits. The combined total number of credits referred and compensated cannot exceed half the taught credits. Decisions with regard to which components should be reassessed are made by the Examination Board. When a student is referred they will normally be permitted to retake the assessment/exam on one further occasion.
At the recommendation of the Board of Examiners, students will normally be allowed one resubmission of a failed dissertation or project and this will normally be within four months of the date of the publication of the result. For September 2016 starters only, failed PGT dissertations can only be re-submitted if they achieve a mark of 30 or above. The Board of Examiners, in agreement with the External Examiner may, exceptionally, decide not to allow resubmission.
The pass mark for a reassessment is the same as the first attempt (i.e. 50% for masters and 40% for Postgraduate Diploma/Certificate).When a reassessment is passed, the mark is capped at the lowest compensatable fail mark (i.e. 40R for Masters and 30R for Postgraduate Diploma/Certificate), unless the previous mark was within the compensation zone, in which case the original mark will stand with a suffix ‘R’. This mark is used in the weighted average/total mark for the final award. The capped mark is applied to the whole unit and not the failed component.
Referrals may also be compensated providing the number of quota of compensations has not been exceeded. When a student’s referral mark is in the compensation zone (and the student/unit is eligible for compensation), the student’s mark will be capped at the lowest compensatable fail mark (i.e. 40R for Masters and 30R for Postgraduate Diploma/Certificate).
Please note that some programmes do not allow referrals. Please refer to the ‘Programme Exemptions to PGT Degree Regulations’ section of the handbook where specific exemptions applicable to the programme will be listed.
Guidance for Presentation of Taught Masters Dissertations
The University of Manchester guidance on presentation of taught Masters Dissertations is available at:
Guidance for the presentation of Taught Masters dissertations
The guidance explains the required presentation of the dissertation, and failure to follow the instructions in the guidance may result in the dissertation being rejected by the examiners.
Turnitin and Plagiarism
Plagiarism and Other Forms of Academic Malpractice
Academic malpractice is any activity - intentional or otherwise - that is likely to undermine the integrity essential to scholarship and research. It includes plagiarism, collusion, fabrication or falsification of results, and anything else that could result in unearned or undeserved credit for those committing it. Academic malpractice can result from a deliberate act of cheating or may be committed unintentionally. Whether intended or not, all incidents of academic malpractice will be treated seriously by the University.
The Faculty of Biology Medicine and Health have designed a learning module to raise your awareness of academic malpractice and how it can occur in general writing during your studies. This resource can be accessed via Blackboard - SMS Introductory Course and must be completed before you submit your first piece of academic writing for assessment.
The University provides workshops and online training via My Learning Essentials: http://www.library.manchester.ac.uk/using-the-library/students/training-and-skills-support/my-learning-essentials/
University of Manchester guidance to students on plagiarism and other forms of academic malpractice is available at:
http://www.regulations.manchester.ac.uk/guidance-to-students-on-plagiarism-and-other-forms-of-academic-malpractice/
The full guidance document can be viewed here: http://documents.manchester.ac.uk/display.aspx?DocID=2870
Academic Malpractice: Procedure for the Handling of Cases can be found at: http://documents.manchester.ac.uk/DocuInfo.aspx?DocID=639
Turnitin
The University uses electronic systems for the purposes of detecting plagiarism and other forms of academic malpractice and for marking. Such systems include TurnitinUK, the plagiarism detection service used by the University.
As part of the formative and/or summative assessment process, you may be asked to submit electronic versions of your work to TurnitinUK and/or other electronic systems used by the University (this requirement may be in addition to a requirement to submit a paper copy of your work). If you are asked to do this, you must do so within the required timescales.
The School also reserves the right to submit work handed in by you for formative or summative assessment to TurnitinUK and/or other electronic systems used by the University.
Please note that when work is submitted to the relevant electronic systems, it may be copied and then stored in a database to allow appropriate checks to be made.
Mitigating Circumstances
Mitigating circumstances are personal or medical circumstances which are unforeseeable and unpreventable that could have a significant adverse effect on your academic performance. You should only submit a mitigating circumstances application if you consider it serious enough, and the timing critical, to have affected your performance in your assessed work and examinations.
Request for mitigation must be submitted to your programme administrator, in advance of your assessment submission deadline or exam. Requests for mitigation submitted after the assessment or exam (except those requests made as a result of circumstances that have arisen during the course of that assessment period) will not be considered without a credible and compelling explanation as to why the circumstances were not known before the beginning of the assessment period or why you were unable to complete or submit an application prior to the assessment or exam. Please note that not informing the University of circumstances due to personal feelings of embarrassment and pride, or having concerns over the confidential treatment of requests for mitigation, are not considered to be credible and compelling explanations
All mitigating circumstances applications must be supported by independent third party evidence. The type of evidence required will vary according to the nature of the circumstances. Examples of evidence include a doctor or other health professional’s letter, counsellor’s letter, self-certification form signed by your GP or GP’s Medical Practice (for illnesses of 7 days and under only). Please note that it is a University policy that the self-certification form must be signed by a GP; we cannot accept forms which have not been signed by a GP. Please note that if evidence has not been received within 2 weeks of the submission of your form, and you have not contacted them to inform them of any delay, your application will be refused and no further action will be taken.
Please ensure that you password protect or encrypt your mitigating circumstances form and supporting evidence before sending to your programme administrator.
Any requests for mitigation will be considered confidentially by a mitigating circumstances panel or sub-panel. Where a request for mitigation is supported, a recommendation will be made to the exam board for them to decide on the best course of action for the student.
You are advised to consult the following guidance, which directs you to seek advice and support before and whilst submitting a request for mitigation.
The University form and guidance for students, is available at: http://www.regulations.manchester.ac.uk/basic-guide-mitigating-circumstances/
For further information about the process and acceptable grounds for mitigation see: Mitigating Circumstances Policy & Procedures: http://documents.manchester.ac.uk/DocuInfo.aspx?DocID=4271
Please be advised that any requests need to be submitted by midday the day before the pre-arranged Mitigating Circumstances meeting.
24th October 2018
28th November 2018
9th January 2019
20th February 2019
20th March 2019
24th April 2019
22nd May 2019
19th June 2019
Late Submission Penalty (Including Dissertation)
Work submitted after the deadline without prior approval shall be subject to the following penalties:
The mark awarded for the piece of work will be reduced by:
· 10 marks if up to 24 hours late (1 day)
· 20 marks if up to 48 hours late (2 days)
· 30 marks if up to 72 hours late (3 days)
· 40 marks if up to 96 hours late (4 days)
· 50 marks if up to 120 hours late (5 days)
A zero mark will be awarded if the piece of work is more than 5 days late.
The sliding scale does not apply to referred assessment, where late submission will automatically receive a mark of zero.
For further information see: Policy on Submission of Work for Summative Assessment on Taught Programmes
Assignment Word Count (including the dissertation)
In accordance with the University Policy on Marking:
Each written assignment has a word limit which you must state at the top of your first page. It is acceptable, without penalty, for you to submit an assignment within a range that is plus 10% of this limit. If you present an assignment with a word count exceeding the specified limit+10%, the assignment will be marked but 1% will be deducted from this mark for every 100 words over the limit given.
For an original word limit that is 1000 words and an assignment that is marked out of 100. If a submission is made that is 1101 words then it exceeded the 10% leeway, and is more than 100 words over the original limit and should receive a 1 mark deduction.
In accordance with accepted academic practice, when submitting any written assignment for summative assessment, the notion of a word count includes the following without exception:
· All titles or headings that form part of the actual text. This does not include the fly page or reference list.
· All words that form the actual essay.
· All words forming the titles for figures, tables and boxes, are included but this does not include boxes or tables or figures themselves.
· All in-text (that is bracketed) references.
· All directly quoted material.
Certain assessments may require different penalties for word limits to be applied. For example, if part of the requirement for the assessment is conciseness of presentation of facts and arguments. In such cases it may be that no 10% leeway is allowed and penalties applied may be stricter than described above. In such cases the rules for word count limits and the penalties to be applied will be clearly stated in the assessment brief and in the submission details for that assessment.
Fitness to Practise
Postgraduate students at The University of Manchester, who are qualified health or social care professionals (e.g. doctor, dentist, nurse, social worker) registered by a healthcare or social care regulatory body (e.g. General Medical Council, General Dental Council, Nursing & Midwifery Council, Social Care Council) are expected to behave at all times, in a way that is consistent with the recommendations, or code of practice, of the relevant professional regulatory body.
Postgraduate students need to be aware that in the event of misconduct, dishonesty, unprofessional behaviour, or other behaviour or illness (e.g. mental health illness) that raises the possibility that the student’s fitness to practise may be impaired, the University has a duty to protect the public and to inform the relevant professional regulatory body.
This means, for example, that where a student has been found to be dishonest (e.g. plagiarism, collusion, falsification of research data or other forms of cheating) the matter may be reported by the University to the relevant professional regulatory body. Students who are dishonest not only risk failing to be awarded the intended degree, but also place at risk their whole professional career.
Information on Fitness to Practise related matters can be found at: www.tlso.manchester.ac.uk/appeals-complaints/fitnesstopractise
Academic Appeals, Complaints, Conduct and Discipline
Academic Appeals
- Students have a right of appeal against a final decision of an Examination Board, or a progress committee, or a graduate committee or equivalent body which affects their academic status or progress in the University.
- Students thinking of appealing should first discuss the matter informally with an appropriate member of staff, in order to better understand the reason for the result or decision.
- Should you wish to proceed to a formal appeal, this must be submitted within the timeframe outlined in the Academic Appeals Procedure to the Faculty Appeals and Complaints Team, Room 3.21, Simon Building, University of Manchester, M13 9PL (e-mail: FBMHappealsandcomplaints@manchester.ac.uk).
- The Academic Appeals Procedure (Regulation XIX) and associated documents, including the form on which formal appeals should be submitted, can be found at regulations.manchester.ac.uk/academic
Student Complaints
- The University’s Student Complaints Procedure (Regulation XVIII) and associated documents, including a complaints form, can be found at www.regulations.manchester.ac.uk/academic
- University has separate procedures to address complaints of bullying, harassment, discrimination and/or victimisation - see https://www.reportandsupport.manchester.ac.uk/
- Students thinking of submitting a formal complaint should, in most instances, attempt informal resolution first (see the procedure). Formal complaints should be submitted on the relevant form to Faculty Appeals and Complaints Team, Room 3.21, Simon Building, University of Manchester, M13 9PL (e-mail: FBMHappealsandcomplaints@manchester.ac.uk).
Conduct and Discipline of Students
- General University information on the Conduct and Discipline of Students can be found at http://www.staffnet.manchester.ac.uk/bmh/teaching/teaching-activity/fitness-to-practise/
- Faculty policies for students on Communication and Dress Code, Social Networking and Drugs & Alcohol can be found at:
- http://documents.manchester.ac.uk/display.aspx?DocID=29038 (Communication and Dress Code)
- http://documents.manchester.ac.uk/display.aspx?DocID=29039 (Drugs & Alcohol)
- http://documents.manchester.ac.uk/display.aspx?DocID=29040(Social Networking)
- Information on Academic Malpractice and how to avoid it can be found at http://www.regulations.manchester.ac.uk/guidance-to-students-on-plagiarism-and-other-forms-of-academic-malpractice/
4. Student Progression
Monitoring Attendance and Wellbeing of Students
The programme director and teaching staff will monitor the work and attendance of students on the programme. This is for your benefit and helps to ensure you are coping with the work. Regular or a pattern of non-attendance and/or engagement will result in you being contacted by the School to meet with your programme director. Following this, further action will be taken if there isn’t a significant improvement in attendance.
For further information see:
Regulation XX Monitoring Attendance and Wellbeing of Students
The University offers a range of advice and support to students experiencing problems with attendance. Further information can be found in the A-Z of Student Services: http://www.studentnet.manchester.ac.uk/crucial-guide/
You can also speak to your Programme Director and/or Academic Advisor.
What to do if you are absent
In case of illness you should supply a doctor’s certificate or, if the illness is brief, a self-certification. If you are absent for other reasons then you should write a letter to the Programme Director explaining the circumstances. Medical certificates or letters should be given in person or sent to the Programme Administrator. Whatever your reason for being away, tell your supervisor about it and make any necessary arrangements to catch up with work you have missed.
Special Permissions
Interruptions to programme and extensions to writing up
It is the expectation of the University that postgraduate taught students pursue their studies on a continuous basis for the stipulated duration of their programme. However, it is recognised that students may encounter personal difficulties or situations which may seriously disrupt or delay their studies. In some cases, an interruption or extension to your programme of study may be the most sensible option.
Students who wish to interrupt the programme or extend to write up the dissertation should initially discuss their plans and reasons with the Programme Director and/or their Academic Advisor.
Students should also provide documentary evidence when appropriate, for example, doctor’s letter, sick note etc.
The forms required for formal application are available from your Programme Administrator.
Tier 4 Visa Attendance Monitoring Census
The University operates attendance monitoring census points within the academic year in order to confirm the attendance of students holding a Tier 4 Student Visa. This is to ensure the University meets the UKVI statutory requirements as a sponsor of Tier 4 students and its responsibilities in accordance with its Highly Trusted Sponsor status.
If you are a Tier 4 visa holder, you must attend these attendance monitoring census points, in addition to complying with your programme’s attendance requirements.
When are the census points?
In the 2018/19 academic year, the attendance monitoring census points will be during the following periods:
- 24th September - 5th October 2018
- 14th January - 25th January 2019
- 15th May - 5th June 2019
- 15th July - 26th July 2019
Please note:
- If you are a new student, registration is your first point to confirm your attendance at the University and you will not be required to attend a separate census point in September/October 2018.
- You will receive an e-mail from your programme administrator to confirm when and where you should go to have your attendance confirmed. You must check your University e-mail account regularly. Failure to check your e-mail account is not a valid reason to be absent from a census point.
What if a Tier 4 student cannot attend a census point?
If you cannot attend in person due to a valid reason which includes: illness; placement; field studies; on year abroad; research work; or any other reason connected to your programme of study, you must email your programme administrator to inform us of your absence and your inability to attend in person. In the case of illness, you must provide a copy of a medical certificate. If you are in this position you should report in person to the School as soon as possible after you return to campus.
Students who are recorded as interrupting their studies are not expected to attend during their period of interruption.
What happens if a student does not attend a census point?
The School must be able to confirm your presence to the UKVI by the end of each census point in the academic year. If you do not attend a census point when required by your School and you do not provide a valid explanation for your absence you will be deemed to be “not in attendance”.
Those students identified as “not in attendance” will be reported to the UKVI and the University will cease to sponsor the student’s Tier 4 visa. The Tier 4 visa will then be curtailed and the student must leave the UK within 60 days.
Further information
For more information on Tier 4 visas: https://www.gov.uk/tier-4-general-visa
If you have any concerns about the attendance monitoring census points, or your Tier 4 visa status, please contact visa@manchester.ac.uk
Withdrawal from the Programme
Students who are considering withdrawing from the programme should discuss this in the first instance with the Programme Director.
If arrangements for withdrawal need to be made, this will be handled by the Programme Administrator, who will manage communication with the Fees and Records Departments and other University bodies as appropriate OR Students may liaise directly with the Programme Administrator who will communicate this information directly to the University Student Services Centre.
5. Student Support and Guidance
Student Support and Guidance
Student support and guidance within the programme
Support and advice is available to all students both formally and informally from the Programme Directors, the Programme Administrator and research project supervisors.
If you have any queries or would like to discuss any issues at all – academic, administrative, technical or personal – please do not hesitate to get in touch. All personal issues will be dealt with confidentially.
If we are unable to help you directly, we can put you in touch with many of the support services that are available to students of the University through our Student Services Centre. You can approach these services independently, without the involvement of programme staff. Use the A-Z of Student Services Guide as an additional source of information.
Student support for the dissertation
During the research project and writing up of the dissertation, students will have individual support from their research project supervisor and scheduled structured sessions to monitor their progress and provide support, with help being offered if any problems are being encountered. In addition, students are made aware that they have the option of contacting the programme directors at any time if they are experiencing difficulties, whether this is in relation to their project, or indeed, with regard to any other issue of relevance.
Student support from the University
The University offers a range of support and guidance services to students, for example, Student Health Service, Student Union Advice Centre, Student Counselling and Careers Advice. Details of all these services can be obtained from the A-Z of Student Services
Counselling Service
The counselling service is available for all students. It is free and consists of a team of professional counsellors. The service provides confidential counselling for anyone who wants help with personal problems affecting their work or well-being.
The service is open 9.00am to 5.00pm Monday to Friday all year round except public holidays.
http://www.studentnet.manchester.ac.uk/counselling/
Occupational Health
Occupational Health is a specialised area of medicine concerned with the way in which an individual’s health can affect his or her ability to do a job and to study and conversely how the work environment can affect an individual’s health. Their aim is to promote the physical, mental and social well-being of students and to reduce the incidence of ill-health arising from exposure to work place hazards.
http://www.occhealth.manchester.ac.uk/
Students Union Advice Centre
The Students Union has advisers who can help with any matter ranging from finances to housing and beyond. On the South Campus, the Advice Centre is on the first floor in the Student Union Building, and is open Monday to Friday, 10.00am to 4.00pm, term time and vacation. There is no need to make an appointment.
Disability Advisory and Support Service (DASS)
The University of Manchester welcomes students with a disability or specific learning difficulties. The University has a Disability Advisory and Support Service, who can supply further information and DASS advisors will be pleased to meet you to discuss you needs. DASS will liaise with your School through the Disability Coordinator to make the necessary arrangements for your support during your time in Manchester.
The DASS office can also provide a copy of the University’s Disability Statement, ‘Opportunities for Students with Additional Support Needs at the University of Manchester’ which sets out the policy and provision for students with a disability.
DASS Contact Details:-
Location: 2nd Floor, University Place
Email: dass@manchester.ac.uk; Phone: 0161 275 7512
Text: 07899 658 790; Website: www.dass.manchester.ac.uk
Disability Coordinator Contact Details:-
Name: Wendy Gregson (0161 306 7972) & Nick Cunningham (0161 275 1344)
Email: dc.medicalsciences.pgt@manchester.ac.uk
Looking after yourself and your patients during Ramadan
The Faculty of Biology, Medicine and Health has produced guidance for healthcare students on fasting and caring: Fasting and Caring - Looking after yourself and your patients during Ramadan: guidance for health care students.
6. Student Representation and Feedback
Student Representation and Feedback
Students, in consultation with the Programme Administrator, should arrange an informal election of their Student Representative near the beginning of the academic year. If more than one person is interested in the role, then each candidate is asked to write a short proposal which is circulated to the student body, and an election is held.
The overall responsibilities of the Student Representative are
- to liaise between staff and students about matters of concern
- to provide two-way feedback on programme and teaching quality
- to promote active student involvement in the development of the programme
- to identify student issues and needs on the programme
- to attend programme committee meetings representing the student voice
- to find effective ways to feedback the outcomes of meetings to the student body
- to attend relevant student representative training
- to liaise with other Student Representatives to gain support and ideas
- to become established as a central point for information and guidance for students in the group.
Student representatives are not required to get involved with fellow students’ personal problems, academic difficulties, or individual student allegations of unfair or inappropriate treatment.
Confidentiality is imperative when dealing with student issues. The representative is chosen by fellow students and has their trust, and must maintain it. Any discussion of an individual student’s situation with a third party requires their consent beforehand.
7. Programme Management
Programme Management and Committee Structure
Programme Management
The programme is managed and operated in accordance with the policies, principles, regulations and procedures of the University of Manchester.
Programme Directors relate to the School and Faculty Postgraduate Teaching Committees on matters relating to admissions, exams, reviews and approval of new programmes and units, quality assurance etc. and policy issues of broad relevance to the Graduate School.
The Programme Committee will meet each semester and consist of the Programme Director, Programme Administrator, Programme Committee members and the unit co-ordinators.
The remit of the committee will be to:
- Oversee the teaching, assessment and examining arrangements;
- Monitor cohort progression including failure rate, withdrawal rate;
- Evaluate the extent to which the learning outcomes are achieved by students;
- Monitor, maintain and enhance standards of all aspects of the programme;
- Evaluate the effectiveness of the curriculum and of assessment in relation to programme learning outcomes;
- Evaluate the effectiveness and relevance of the teaching and learning methods employed;
- Review and revise the programme in the light of any relevant Quality Assurance Agency (QAA) benchmarks, any other relevant external and/or professional requirements and developing knowledge in the subject area;
- Receive, consider and respond to feedback from students, employers and external examiners;
- Where the need for change is identified, effect the changes quickly and efficiently;
- Produce an annual action plan via annual monitoring;
- Produce reports for periodic review
- Produce relevant information for an Institutional Audit;
- Review programme documentation, e.g., programme handbooks, programme specifications, promotional literature and programme website;
- Ensure suitable and efficient arrangements are in place for recruitment, admission and induction.
Committee Structure
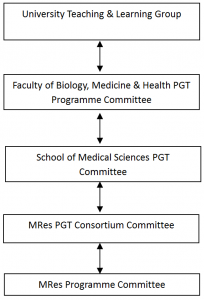
The Programme Committee acts as a curriculum development team for the Programme. The Programme Committee will report to a School, or Department, or Faculty level committee. The Programme Director is responsible for the management of the programme, and the Programme Committee is established to support the Programme Director in the carrying out of their responsibilities.
The role of the External Examiner
External Examiners are individuals from another institution or organisation who monitor the assessment processes of the University to ensure fairness and academic standards. They ensure that assessment and examination procedures have been fairly and properly implemented and that decisions have been made after appropriate deliberation. They also ensure that standards of awards and levels of student performance are at least comparable with those in equivalent higher education institutions.
External Examiners’ reports
External Examiners’ reports relating to this programme will be shared with student representatives at the programme committee, where details of any actions carried out by the programme team/School in response to the External Examiners’ comments will be discussed. Students should contact their student representatives if they require any further information about External Examiners’ reports or the process for considering them.
External Examiner Details
The Programme External Examiner for this programme is Glen Clack (subject to approval).
The Subject External Examiner – TBC
Please note that it is inappropriate for students to make direct contact with External Examiners under any circumstances, in particular with regards to a student’s individual performance in assessments. Other appropriate mechanisms are available for students, including the University’s appeals or complaints procedures and the UMSU Advice Centre. In cases where a student does contact an External Examiner directly, External Examiners have been requested not to respond to direct queries. Instead, External Examiners should report the matter to their School contact who will then contact the student to remind them of the other methods available for students. If students have any queries concerning this, they should contact their Programme Office (or equivalent).
8. Learning Resources
Learning Resources
Libraries
All registered students may become members of the University of Manchester Library on the main campus.
Up-to-date news about the library is available here.
IT Services and eLearning
IT Services Support Centre online
Details can be found at: http://www.itservices.manchester.ac.uk/help/
Login to the Support Centre online to log a request, book an appointment for an IT visit, or search the Knowledge Base.
Telephone: +44 (0)161 306 5544 (or extension 65544). Telephone support is available 24 hours a day, seven days a week.
In person: Walk-up help and support is available at the Joule Library, Main Library or Alan Gilbert Learning Commons:
Use Support Centre online for support with eLearning, from where you may make a request, report a fault, or search the Knowledge Base. The email address is: elearning@manchester.ac.uk
Blackboard
Blackboard, the University's 'virtual learning environment', will be used for online teaching.
What is Blackboard?
Blackboard is a web-based system that complements and builds upon traditional learning methods used at The University of Manchester. By using Blackboard you can
- view course materials and learning resources,
- communicate with lectures and other students,
- collaborate in groups,
- get feedback
- submit assignments
- monitoring your own progress at a time and place of your own convenience.
Training in the use of software
The Faculty eLearning team have produced a short introduction to Blackboard for new students. The recording is hosted in two places: the VLS and on YouTube:
The recording is just over seven minutes long and covers most of the commonly used tools in Blackboard.
9. Useful Links
Academic and Student Support Policies
Academic Support Policies
A list of University Policies and documents can be found at:http://documents.manchester.ac.uk/list.aspx
Academic Appeals (Regulation XIX)
http://documents.manchester.ac.uk/DocuInfo.aspx?DocID=187
Academic Malpractice: Procedure for the Handling of Cases
http://documents.manchester.ac.uk/DocuInfo.aspx?DocID=639
Basic Guide to Student Complaints
http://documents.manchester.ac.uk/display.aspx?DocID=23875
Conduct and Discipline of Students (Regulations XVII)
http://documents.manchester.ac.uk/DocuInfo.aspx?DocID=6530
General University information on the Conduct and Discipline of Students can be found at www.tlso.manchester.ac.uk/appeals-complaints/conductanddisciplineofstudents/.
Faculty policies for students on Communication and Dress Code, Social Networking and Drugs & Alcohol can be found at:
- http://documents.manchester.ac.uk/display.aspx?DocID=29038 (Communication and Dress Code)
- http://documents.manchester.ac.uk/display.aspx?DocID=29039 (Drugs & Alcohol)
- http://documents.manchester.ac.uk/display.aspx?DocID=29040(Social Networking)
Information on Academic Malpractice and how to avoid it can be found at
http://www.regulations.manchester.ac.uk/guidance-to-students-on-plagiarism-and-other-forms-of-academic-malpractice/
Data Protection
http://www.manchester.ac.uk/aboutus/documents/privacy/
Guidance for the Presentation of Taught Masters Dissertations
http://documents.manchester.ac.uk/display.aspx?DocID=2863
Guidance to Students on Plagiarism and Other Forms of Academic Malpractice
http://documents.manchester.ac.uk/display.aspx?DocID=2870
Policy on Submission of Work for Summative Assessment on Taught Programmes
http://documents.manchester.ac.uk/display.aspx?DocID=24561
Policy on Mitigating Circumstances
http://documents.manchester.ac.uk/display.aspx?DocID=4271
Mitigating Circumstances Guidance for Students
http://documents.manchester.ac.uk/display.aspx?DocID=23886
PGT Degree Regulations
http://documents.manchester.ac.uk/display.aspx?DocID=29208
Policy on Feedback to Undergraduate and Postgraduate Taught Students
http://documents.manchester.ac.uk/DocuInfo.aspx?DocID=6518
Student Complaints Procedure
http://documents.manchester.ac.uk/DocuInfo.aspx?DocID=1893
Student Charter
http://www.studentnet.manchester.ac.uk/enhancing-my-experience/charter
Work and Attendance of Students (Regulation XX)
http://documents.manchester.ac.uk/display.aspx?DocID=1895
A-Z of Student Services
http://www.studentnet.manchester.ac.uk/crucial-guide/
Accommodation
http://www.accommodation.manchester.ac.uk/
Blackboard
Students should access Blackboard via my Manchester at https://my.manchester.ac.uk
Careers Service
http://www.careers.manchester.ac.uk/
Counselling Service
https://www.counsellingservice.manchester.ac.uk/
Disability Advisory and Support Service
http://www.dass.manchester.ac.uk/
University Language Centre – Study English - Tel: 0161 306 3397
http://www.languagecentre.manchester.ac.uk/study-english/
Equality, Diversity and Inclusion for Staff and Students
http://www.manchester.ac.uk/aboutus/equalityanddiversity/
Health & Fitness
http://www.sport.manchester.ac.uk/
Health & Safety Policy
http://documents.manchester.ac.uk/display.aspx?DocID=654
International Advice Team
http://www.manchester.ac.uk/international/support/advice/
IT and eLearning Support
http://bmh-elearning.org/technical-support/
Mature Students Guide
http://documents.manchester.ac.uk/display.aspx?DocID=18122
Occupational Health Services for Students
http://www.occhealth.manchester.ac.uk/postgraduates/
Personal Development Planning
http://www.tlso.manchester.ac.uk/personaldevelopmentplanning/
A Personal Safety Guide for International Students
http://www.studentnet.manchester.ac.uk/medialibrary/study/safety-international-student-guide.pdf
Students Union
http://manchesterstudentsunion.com/
10. Appendix
Good Research Conduct
Code of Good Research Conduct
http://documents.manchester.ac.uk/display.aspx?DocID=2804
Good Research Practice Booklet
http://www.researchsupport.eps.manchester.ac.uk/documents/policy/UOMgoodresearchpractice.pdf
General principles
This code is written to preserve the highest professional standards, while striving to maintain an environment that values creativity and flexibility.
- All work must be carried out in accordance with the highest standards of scientific practice.
- Policies on safeguarding good scientific practice are available from the BBSRC at http://www.bbsrc.ac.uk/web/FILES/Policies/joint_code_of_practice_for_research.pdf
Extract from Good Research Practice Booklet
3.9 Recording, Storing and Archiving
Research Data/Materials
As leader of a research project, you are responsible for ensuring that there are clear protocols for the collection, recording, storage and archiving of research data/materials generated as part of your project. These protocols should fit within any professional guidance available, guidance from funding bodies, your school and the University’s Code of Good Research Conduct.
3.10 Health and Safety
It is your responsibility to ensure that the research staff and students for whom you have responsibility are provided with an environment that is safe and healthy and all research is conducted within the requirements of health and safety legislation:
- That necessary risk assessments have been undertaken (Never assume that because your research is not lab-based or using hazardous substances that it would not require a risk assessment).
- That staff are adequately informed, trained and monitored regarding safe practices to ensure they do not endanger themselves, others or the environment.
- That your research complies with the Control of Substances Hazardous to Health regulations as appropriate.

